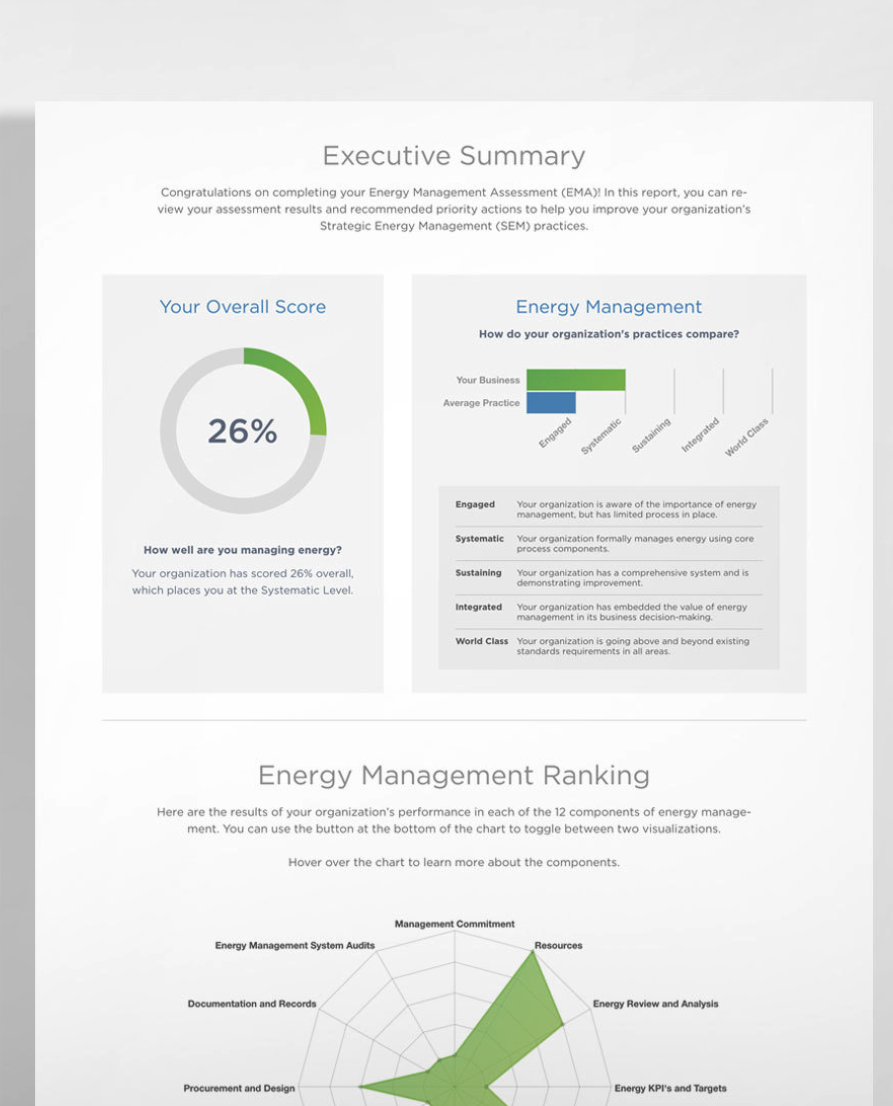
comirnaty public assessment report - en : EMA - European Medical Association : Free Download, Borrow, and Streaming : Internet Archive

European Public Assessment Report (EPAR) summaries for the public: are they fit for purpose? A user-testing study | BMJ Open

Study selection. Flow chart shows the selection process starting with... | Download Scientific Diagram
Improving the Contribution of Regulatory Assessment Reports to Health Technology Assessments—A Collaboration between the Europ

Improving the Contribution of Regulatory Assessment Reports to Health Technology Assessments—A Collaboration between the European Medicines Agency and the European network for Health Technology Assessment - ScienceDirect
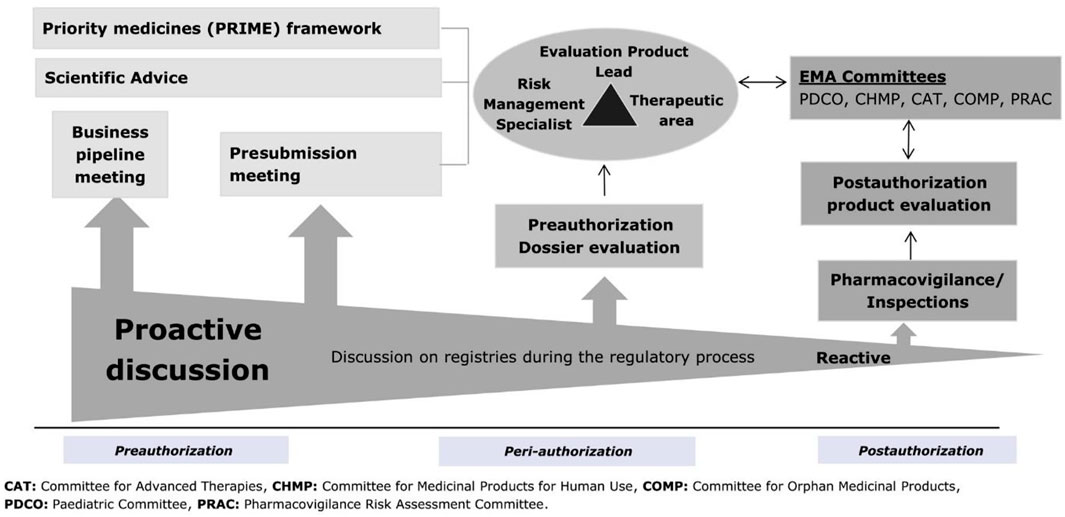
Frontiers | Contribution of patient registries to regulatory decision making on rare diseases medicinal products in Europe